6TH DENGUE EXPERTS MEETING OF THE INTERNATIONAL DENGUE INITIATIVE FOR IMPLEMENTATION OF THE DENGUE VACCINE IN LATIN AMERICA.
INTRODUCTION
The 6th Experts meeting on dengue vaccine implementation in Latin America was held in Lima, Peru on 22 and 23 June 2018.
The meeting convened a permanent group of Latin American and other international experts in dengue and vaccination. This group was endorsed by the regional societies SLIPE (Latin American Society of Pediatric Infectology) ALAPE (Latin American Association of Pediatrics) and API (Pan American Association of Infectology).
The objective of the meeting was to update the International Dengue Initiative Group Position Paper on dengue vaccination in Latin America, based on the new manufacturer product label and SAGE recommendations, as well as discussions on relevant concepts, the latest evidence, and implementing country experiences.
This meeting included a series of lectures, workshops and discussions. The current report summarizes the main conclusions and the group position.
- BACKGROUND INFORMATION
- THE DISEASE
According to the WHO, Dengue is considered to be a worldwide threat to public health (1); half of the population lives in areas at risk of dengue, representing a global epidemic threat. Since the 1980s, the number of dengue cases reported in the Americas has doubled each decade, reaching nearly 13 million accumulated cases since 2010. In 2015-2016, the number of cases increased drastically, followed by an unexplained drop in 2017-2018, according to the cyclical behavior of the disease and with the accumulation of the susceptible population, an important outbreak is expected in the region in the years to come. Lethality in the region is variable (2,3).
The true burden of disease of dengue is greatly underestimated, with real incidence rates that could be 10 to 20 times higher than reported in some countries. (4, 5, 6, 7, 8, 9, 10).
The pathogenesis of severe dengue is not fully understood, it is complex; it appears to be the result of interaction between different factors, such as the level of antibodies, their type and quality, cellular response, the serotype, particularly the genotype, of the virus, as well as the age and the individual characteristics of the host ( 11-22).
- KEY ASPECTS TO CONSIDER ABOUT The DENGUE VACCINE.(23-28)
- Phase III Study Results:
Summary of the characteristics of the only vaccine currently available for the prevention of dengue according to the age of indication, in agreement with the data from the combined analyses of the phase III studies
- Efficacy
Against virologically-confirmed dengue (VCD): 65.6%
Against severe disease and hospitalization due to VCD it was 92.9% and 80.8% respectively.
Per serotype:
- Serotype 1: 58.4% (47.7 to 66.9)
- Serotype 2: 47.1% (31.3 to 59.2)
- Serotype 3: 73.6% (64.4 to 80.4)
- Serotype 4: 83.2% (76.2 to 88.2)
Efficacy according to the previous serostatus:
- Seropositive: 81.9
- Seronegative:52.5 (Statistically significant)
- Data from Long Term Follow Up:
The R.R. of hospitalization in long-term follow-up in a pooled analysis of phase III studies was 0.374 (0.30 to 0.47) in the target population, regardless of the country or previous serostatus, demonstrating a permanent population benefit throughout over time.
The LTFU data, according to previous serostatus evaluated in the immune subset (2 thousand subject in each phase III Study) do not show an increased risk of hospitalized or severe dengue at any time point in seronegatives over 9 years of age, but data is limited due to the number of cases of hospitalized and severe dengue that occurred in the immunogenicity subset, for which baseline serostatus was known.
The Scientific, Public Health and Regulatory communities have indicated that there is an important knowledge gap in the CYD vaccine safety among the seronegative population, and that very limited information was available from the efficacy studies, due to the small size of the immunogenicity subset.
2. Post Hoc Analysis, to evaluate the effect of previous immune status (26,27):
Sanofi Pasteur has developed a Post hoc analysis that intended to assess the CYD-TDV performance according to previous immune status. This analysis is based on a case-cohort study derived from the phase III and IIb studies
- In the absence of samples at baseline for all the participants of the studied population, samples from month thirteen (one month after the third dose) available from each of the 31,000 participants, were used to estimate previous dengue serostatus.
- A recently developed dengue anti–nonstructural protein 1 (NS1) IgG enzyme-linked immunosorbent assay (ELISA) was used to differentiate between anti-NS1 antibodies induced by wild-type dengue infection and those induced by vaccination, since CYD-TDV contains genes encoding NS1 from the yellow fever 17D vaccine virus instead of the dengue virus.
- The investigators used different approaches to define serostatus (The full methodology is described in Sridhar et al, 2018 published in the NEJM).
- In order to avoid misclassification due to asymptomatic disease during the first 12 months or vaccine effect due to cross reactivity, two imputation methods were used: A logistic regression for multiple imputations and a super learner for targeted minimum loss–based estimation. For the principal analysis, a baseline serostatus was determined on the basis of measured or imputed titers from a 50% plaque-reduction neutralization test (PRNT50) was used.
- The percentage of subjects with a missing PRNT 50 at Mo was 66.7%.
- Main results:
- In seropositive participants aged 9-16 years:
- In the active phase (up to month 25), vaccine efficacy was 76% ) against symptomatic VCD.
- The relative risk after 60 months of follow-up was 0.2 for hospitalization and/or severe dengue, and 0.14 for severe dengue.
- In seronegative subjects of the same age group:
- The corresponding values for V.E were 39% (95% CI, −1 to 63), 1.40 (95%CI, 0.70 to 2.80), for hospitalization and 2.29 (95%CI, 0.44 to 11.92) for severe dengue.
- None of the data reached a statistical significance.
- Vaccinated seronegative subjects developed less hospitalized VCD than non-vaccinated subjects until month 30; then the curve crossed over the control curve and slowly caught up with the seropositive control curve over the following months.
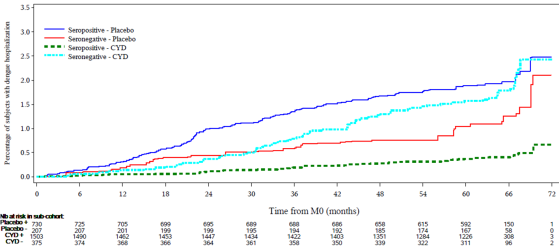
- The similar incidence of hospitalization and severe dengue in vaccinated seronegative trial participants and unvaccinated seropositive participants is consistent with the hypothesis that vaccination in seronegative individuals causes a primary-like infection (24, 25). It seems plausible that CYD-TDV mimics the immune response to primary wild type infection in seronegative subjects; therefore, due to the waning of protection, the subject could be at the same risk of wild type secondary infection after 30 months of vaccination than those suffering from natural primary infection.
Although a cohort case study in a post hoc analysis is valid from the methodological point of view, the reported low power (25% for an RR of 1.4, with an estimated 72 cases, in a seronegative population over 9 years old) (27), the difficulties to define the serological status at the baseline based in the month 13 data and the limitations of the imputation per se, this study could penalize a good vaccine to prevent dengue in populations over 9 years old.
- Implications of these findings in public health: (24-28)
- At a population level, there is a marked reduction in the attributable risk of hospitalized and severe dengue with vaccination of seropositive subjects aged 9−16 years old. Based on attributable risk estimations over a 5-year follow-up period, for each additional case in vaccinated seronegative subjects, the numbers of hospitalized and severe cases prevented in vaccinated seropositive subjects would be 7 and 4 respectively in 70% seroprevalence settings, 13 and 7 in 80% seroprevalence settings, and 28 and 16 in 90% seroprevalence settings.
- The number needed to vaccinate (NNV) is a public health surveillance outcome expressing how many people need to be vaccinated to prevent a single disease outcome.
- To prevent one case of hospitalization with VCD, 91 subjects need to be vaccinated (NNV=91), while NNV equals 400 for severe dengue. In comparison with the influenza vaccine NNV=1850 for hospitalization in children, and with the meningococcal MenB vaccine NNV=35,000 for meningitis. Therefore, the public health value of the dengue vaccine as estimated by NNV is similar or better than other vaccines.
- This vaccine, as all others, needs to be evaluated in the context of an impact on public health.
3. NEW SAFETY DATA (25,30,31)
- There are no differences in the incidence of severe adverse events (SAE) other than dengue and fatal SAEs after 5 years of follow-up (LTFU) in subjects of the vaccinated group and the placebo group, in both seronegative and seropositive.
Regarding the post marketing experience, in a safety analysis that was recently published by the Global Advisory Committee on Vaccine Safety (GACVS), dengue deaths in the Philippines were evaluated and it was concluded that, in accordance with the WHO classification of adverse events in vaccines, most cases are indeterminate, coincidental (unrelated) or unclassifiable. There is not enough evidence to link the CYD-TDV with dengue deaths in the Philippines.
Safety data of the dengue vaccine in Parana, Brazil (32,33)
- In Parana, the community-based dengue vaccination campaign started in August 2016. Thirty municipalities were selected, based on disease incidence criteria and past epidemics. Based on the dengue burden of disease by age identified by the Parana State Surveillance System, the vaccine was administered to 15 to 27-year-old subjects in 28 municipalities, and 9 to 45-year-old subjects in 2 municipalities, with a total target population of 500 000 inhabitants. Three doses were administered at 6-month intervals.
- In total, 639 579 doses were administered with a vaccine coverage around 60% for the first dose (311 058 vaccines), 70% (219 078 vaccinated) for the second dose, and 50% for the third dose (25, 26).
- The databases for vaccination (the Vaccine Registration System) and Dengue Surveillance (SINAN) were linked in order to compare vaccinated individuals with confirmed dengue cases. In 2017, 311 053 subjects were vaccinated in Parana, of whom 49 (0, 01%) had confirmed dengue. None of these cases required hospitalization. The total number of confirmed dengue cases reported in the population of Parana was 1108 out of which 47(4.2%) were vaccinated.(25,26)
- Adverse events following immunization were notified by email or directly to the public health unit. There were no deaths related with the vaccine. The majority of the adverse events were mild and the serious AEs were not related to vaccination. Although no data on vaccine impact is yet available, the incidence of dengue decreased in the 30 municipalities and those with better coverage showed a lower incidence of dengue (32).
C. A DENGUE SEROTEST AS A COMPLEMENTARY DIAGNOSTIC TEST TO SUPPORT VACCINE IMPLEMENTATION (34)
The need for a diagnostic test capable to quickly detect past dengue infections has emerged.
Ideally, a test with very high specificity (≥99%) would minimize individual risk and the inadvertent use of the vaccine in seronegative people. A test with a high sensitivity (≥90%) would maximize the benefit for the population.
Two types of tests could be considered:
1. Serological assays such as the dengue IgG ELISA
- The rapid diagnostic tests (RDT), based on the detection of antibodies against dengue-specific antigens.
The ELISA assays do not provide point-of-care information on an individual’s serostatus.
Sanofi Pasteur developed a preliminary evaluation of rapid diagnostic tests (RDTs) and conventional enzyme-linked immunosorbent assays (ELISA) to determine prior dengue infection, the main results show:
- A favorable specificity >98% for IgG component of RDTs and ELISA,
- Sensitivity was variable from 40-70% for RDT and 90% for ELISA.
- The false positive rates due to Japanese Encephalitis or Yellow Fever cross reactivity was ≤3%, especially for RDT, and ELISA (Panbio), but not for ELISA (focus).
- The high specificity of these available assays despite the existing limitations in sensitivity allows their use to be considered in endemic settings until better tests become available. (25)
D. MODELING BASES FOR THE IMPLEMENTATION OF THE VACCINE (35,36)
Sanofi Pasteur has developed a yet unpublished model-based approach using the NS1 results, to assess benefits and risks associated with dengue vaccination in different transmission settings and time horizons, and considering indirect protection or not. ( Coudeville L et al, presentation during the IDI meeting)
The leading hypothesis: Vaccination mimics a silent natural infection and modifies the probabilities of disease outcomes in the same manner as a natural infection.
Consequences according to serostatus prior to vaccination
- Seropositive subjects: Long-term and sustained benefit
- Seronegative subjects: Transitory period of risk due to higher severity of secondary infection, vaccination becomes beneficial for seronegative subjects that have at least 2 infections.
Main driver for efficacy is the baseline serostatus, and other drivers are age at the time of vaccination and serotype.
Potential indirect benefits of vaccination include an improvement of vaccination impact for seronegative subjects due to a reduction in the risk of dengue for unvaccinated individuals.
The analyses indicate that the population living in areas with a seroprevalence exceeding 50% before age of 20 y.o ranges from 145 to 160 million people in Brazil, and from 35 to 45 million people in Mexico. Similarly, the population living in areas with a seroprevalence exceeding 80% before age 20 ranges from 95 to 116 million people in Brazil, and from 10 to 22 million people in Mexico.
The WHO considered 2 approaches for the implementation of the dengue vaccine:
- A seroprevalence-based strategy: Vaccination regardless of dengue serostatus in high transmission settings (> 80% seroprevalence at age 9).
- A pre-vaccination screening strategy: Vaccination of subjects testing positive for prior dengue infection or an antecedent of a confirmed Dengue case. This is the preferred WHO SAGE option.
The potential impact of dengue vaccination was evaluated with and without pre-vaccination screening in various transmission settings and using current (sensitivity 70%; specificity 99%) or optimized (sensitivity 90%; specificity 99%) RDT.
With pre-vaccination screening, the impact depends on test sensitivity for detecting past dengue exposure.
Pre-vaccination screening enables implementation of larger programs associated with higher impact, unless a large portion of the population is known to live in high transmission settings.
In moderate transmission settings (50% at age 9), pre-vaccination screening is more efficient (and potentially more cost-effective) than a seroprevalence-based approach, provided that the screening cost is lower than the vaccine cost (< 75% of the cost of administering a vaccine dose).
In very high transmission settings (90% at age 9), seroprevalence-based vaccination remains more efficient than pre-vaccination screening (unless serotesting is very inexpensive i.e. 10% or less of the cost of administering a vaccine dose).
In conclusion, in known high transmission settings (≥ 80% seroprevalence at age 9), seroprevalence-based vaccination is associated with the largest impact, and is expected to be more cost-effective than pre-vaccination screening. In other settings or in the context of heterogeneous transmission, pre-vaccination screening enables the implementation of larger vaccination programs having a broader impact on health; it is potentially more cost-effective than the seroprevalence-based approach if testing is not too costly, and it reduces the risks for seronegative individuals. A RDT optimized for pre-vaccination screening should improve the impact and cost-effectiveness of dengue vaccination programs
ii. International Dengue Initiative (IDI) RECOMENDATIONS.
- The disease should be documented in each country and sub-region, in terms of:
1.Endemicity (specific areas where the disease occurs continuously and with a predictable regularity in a population (38) or sustained notification of dengue cases for 20 weeks or more in at least one of the last 5 years.
2. Severity
3. Burden of disease by age group
B. Each country needs to work in its own dengue transmission map according to its epidemiological data (incidence, age of incidence and of hospitalization, serotypes circulation, frequency of outbreaks).
C. In order to define high transmission areas, it could be useful to use the following criteria:
- At least 2
dengue epidemics during the last 5 years
- Cumulative incidence over 500 per 100,000 inhabitants over the last 5 years.
- Reports of dengue deaths in at least one of the past years.
- Co-circulation of at least 2 serotypes.
- High hospitalization rates in adolescents.
D. Many countries currently have small-scale detailed maps describing the epidemiology of dengue over the past years, including seroprevalence data. These maps allow prioritizing vaccine intervention areas, which should be started as soon as possible.
- For seroprevalence studies, we highly recommend using guidelines developed by the WHO to design and conduct dengue serosurveys (37).
- The vaccine is an important component of an integrated strategy for dengue prevention and control. Other preventive measures such as vector control, prevention of mosquito bites, training and information need to be maintained. Research activities on prevention measures need to be continued.
- The characteristic of dengue transmission in the target population should be evaluated in advance so as to discriminate between mass vaccination and vaccination of seropositive individuals.
- In areas of high endemicity in which seropositive subjects predominate, the benefit clearly outweighs the risk at a population level. The studies for seroprevalence or the pre-vaccination serologic assessment would have several limitations; they would significantly increase the costs of vaccination programs, delaying the decision of vaccine introduction into areas with a high burden of disease that would potentially benefit from vaccination programs.
- In areas with high seroprevalence (above 80%), pretesting would not add any benefits and would reduce the cost effectiveness of the vaccination strategy.
- Countries can start vaccination in municipalities that already have seroprevalence studies and that fulfill the criteria.
- In areas of intermediate or low endemicity, where the benefit-risk balance is not clear, pretesting to establish the patient’s history of infection before vaccination is mandatory. This strategy allows better vaccination coverage, as well as the generation of seroprevalence data allowing subsequent decision taking.
L. Specific considerations for pretesting:
- The ELISA capture test may not be practical due to the time it takes to obtain results. In addition to having cross reactivity with other flaviviruses, it should not be recommended in countries with high co-circulation of flavivirus.
- RDTs could be implementable at point-of-care and need to be friendly, qualitative, use all the blood, and be validated to indicate past dengue infection at any age, in any endemic setting.
- The ideal test needs to be highly specific in order to avoid vaccination of seronegative subjects and have a high level of sensitivity in order to maximize the impact vaccinating a high number of seropositive subjects.
- A reasonable option would be to use a test with the highest specificity currently available, and in the meantime develop optimized rapid diagnostic tests.
- When a test is required for serostatus confirmation, the consensus is not to delay vaccination where it is needed while waiting for the next generation RDT.
- Countries should use the best available tests; help develop new ones by sharing epidemiological data and biological samples, and conduct demonstration projects with current tests.
M. The vaccine is not indicated as a response to outbreaks but to help preventing an outbreak from occurring.
N. Countries should implement a robust and documented vaccination information strategy, and optimal program planning. HPV vaccination strategies can serve as examples, and lessons learnt from HPV vaccine implementation should help dengue vaccination.
O. Vaccine-implementing countries should have a robust surveillance of adverse events.
P. Committees should be strengthened and adequate information given to those in charge of the program.
Q. Surveillance should include the number of doses given, the epidemic situation, and confusion factors.
R. Age:
- Currently the vaccine is indicated in populations older than 9 y/o.
- The age of implementation needs to be in accordance with local regulatory agency recommendation.
- For public campaigns, the age targets for vaccination should be in the age groups with a higher seroprevalence or a higher incidence of hospitalization.
- Selection of the target age group must be in accordance with the previous experience in vaccine adherence.
- A catch up campaign could be implemented in order to have a higher and faster impact. The extension of the catch-up campaign will depended on modeling information using local data for an optimal impact.
S. Countries are empowered to take their own decisions based on evidence-based information and support from various panels of experts.
T. The guidelines developed by scientific and medical societies (e.g. SLIPE) should be given more visibility and should help country’s decision-making process.
U. In summary, the vaccine has to be implemented as a public health strategy and vaccine introduction requires the consensus of scientific societies, the MOH and the population to guarantee the implementation of an adequate communication program. V. The recommendations need to be updated regularly, as soon as new scientific evidence comes to light.

